We, as endurance athletes in the world of the COVID-19, indoor-training dominated world, are perhaps more than ever having to make day-to-day decisions on how and when we should train, and how and when we should race. Many of us have had our A and B races canceled this year, or have had to shift our training environment indoors, and, almost inevitably, onto Zwift. Zwift is an awesome tool for keeping us motivated and occupied from within the confines of our indoor pain caves, and Zwift racing is an exciting means of keeping those competitive juices flowing while the finish line in Kona seems further and further away.
Imagine an athlete setting up on their trainer for an after-work spin on a Friday. They have a swim and some intervals planned on the bike for Saturday, followed by a long endurance ride on Sunday and an 8-km run-off-the-bike. They see that there’s a Zwift race starting in 20 min they fancy having a crack at. Should they do it? How do they make the decision? Having an understanding of the physiology of Zwift racing, and its ensuing recovery, should help with your decision-making here.

Physiology of the Zwift race
Zwift cycling races come in various shapes and sizes, but they are most commonly ~10-20-km in distance, or around ~20-30 min in duration. Whilst we typically see high average power outputs and heart rates over the whole race, these events are quite stochastic or variable in their intensity, characterized by brief periods of very high intensity work, and short periods of lower outputs. They are like a condensed version of a road race, typically with a very intense start that goes a long way to deciding the outcome (or at least determining who won’t win). Have a look at the power and heart rate curves below, taken from an athlete with an anaerobic threshold of ~360 W and ~143 b.min-1 during a Zwift race over 36 km.
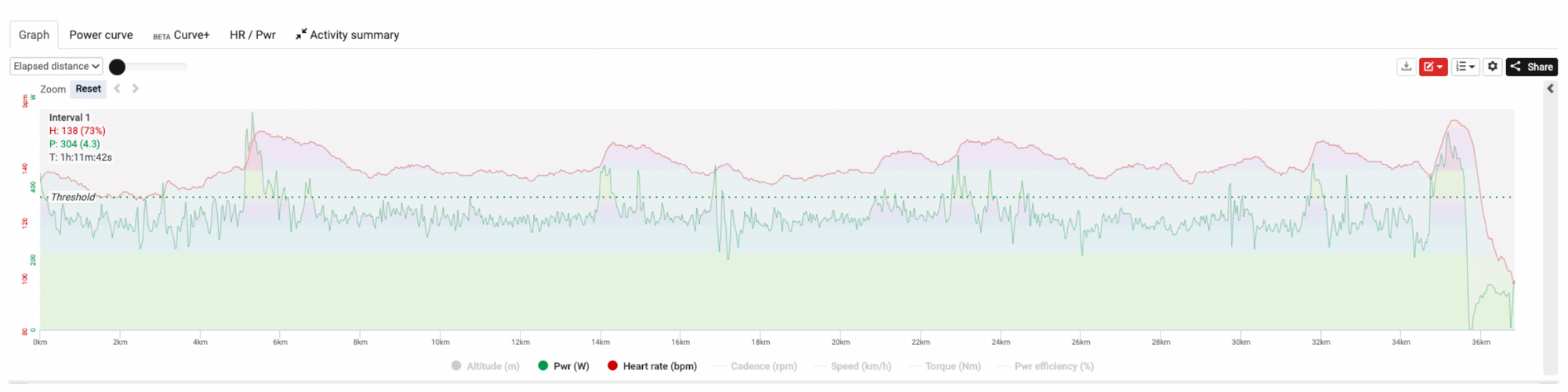
As you can see, these races evoke a fair physiological load, with segments of time above anaerobic threshold and into the severe intensity domain. From a physiological perspective, this means that by the finish intramuscular stores of phosphocreatine (PCr) will likely be quite depleted (4), and there will be a pronounced acidosis in the muscle and therefore in the blood (7). The significant period of high intensity work will require high circulation of stress hormones (sympathetic drive) to kick us into gear metabolically (13), and we will have partially depleted our muscle glycogen energy stores (14). Whilst great fun and probably a fairly good stimulus for adaptation, these types of sessions are highly stressful on our physiology.
Physiology of recovery
How long does that stress take to get over? Are we going to be fresh for the swim-bike 12 hours later? The figure below shows the time-course of recovery of a range of physiological variables following a high-intensity training session like a Zwift race.
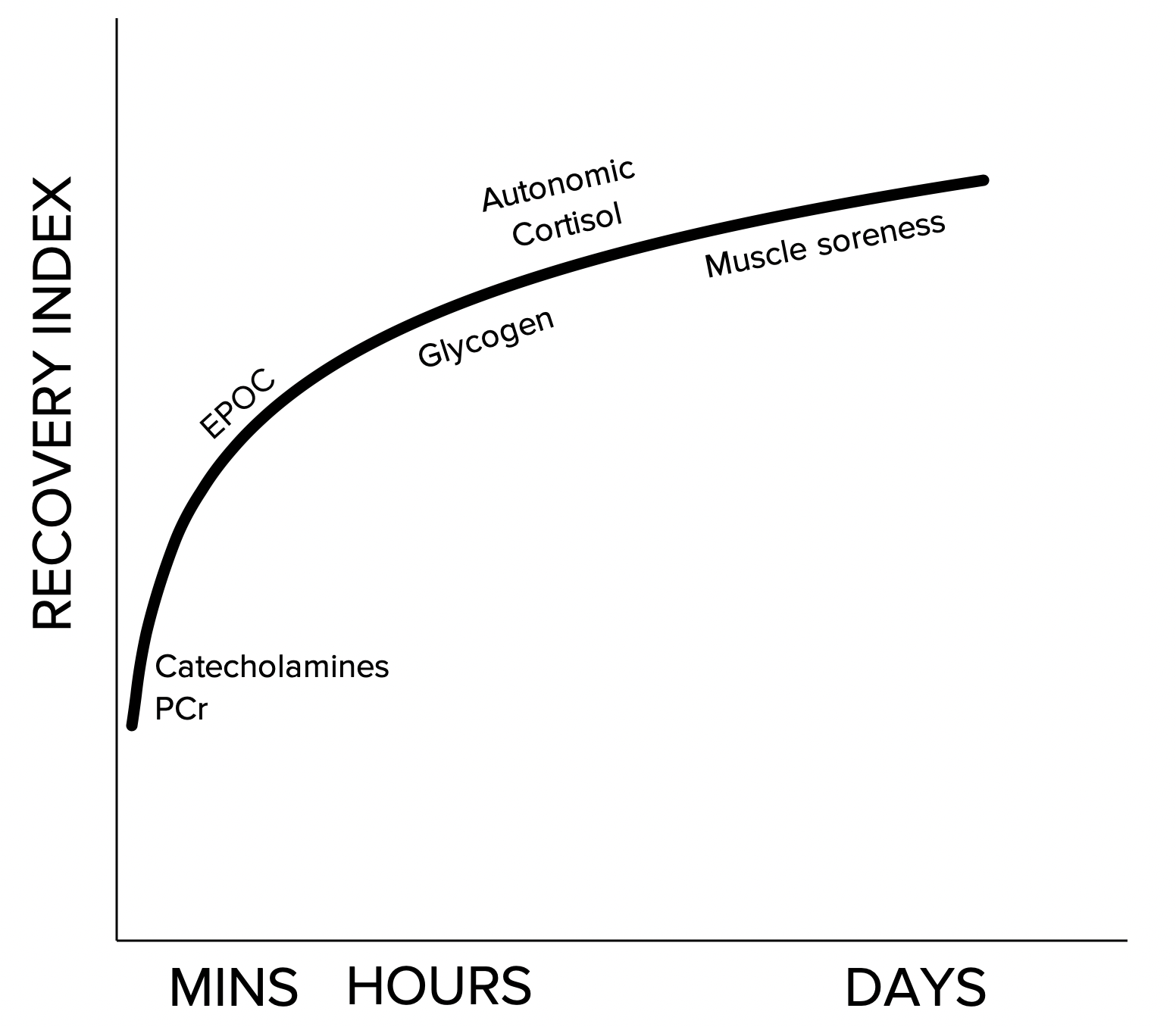
As you can see above, physiological variables like intramuscular PCr stores, which are extremely important for the metabolism of sprint exercise, recover in the minutes that follow a given high-intensity session (4). PCr availability is actually very fluid even during a bout of stochastic high-intensity exercise, with PCr depleted during periods of high-intensity work and then recovered during ensuing periods of recovery below critical power or the ‘anaerobic threshold’ (6). Catecholamines, the stress hormones adrenaline and noradrenaline, will return to normal very quickly in the period following high-intensity exercise (17), whilst oxygen consumption (VO2) is elevated in the hours following exercise to a magnitude dependent on the intensity and duration, reflecting how recovery from exercise places greater demand on the aerobic energy system than normal rest (11). Muscle glycogen stores can be replenished back to near baseline concentrations in a period of hours, though the rate at which this replenishment occurs will be dependent on the amount and type of carbohydrate consumed, and the magnitude of the depletion induced by the exercise session (1). Autonomic balance, i.e. physiological stress as indicated by sympathetic nervous system activity and heart rate variability (HRV), is likely to be disturbed for a period of hours, perhaps 20-40, after a high-intensity session (10). Finally, perceived muscle soreness after a strenuous training session or race can leave its mark for days following exercise (8), although this is more of a concern for running than cycling, particularly if it includes any downhill work. Running downhill requires eccentric or lengthening muscle contractions, which essentially provide a braking force as you resist gravity. Eccentric contractions evoke more muscle damage than concentric contractions – cycling is a largely concentric action (2) – and will therefore likely leave you with more soreness (5). Indeed, downhill running is a common methodology used in studies to induce muscle damage and soreness for research purposes (5).

This recovery time-course in contrast to that following the specific low-intensity training that forms the base of most good endurance training programs (~70-80% of training time) (3, 12, 15, 18, 19, 22), which specifically evokes low physiological stress and therefore shouldn’t leave athletes feeling as jaded the next morning (10, 20), allowing accumulation of the substantial overall training volumes required for high performance. Indeed, autonomic disturbance is minimal following low-intensity training below the aerobic threshold (20). Although there are large variations between individuals, glycogen may be depleted to some extent after a low-intensity session; particularly if the duration is long.
Therefore, following a high but stochastic-intensity, short(ish) duration Zwift race, you are likely to see marked reductions in muscle PCr stores, as well as pH, though these will recover in minutes. Stress hormones will recover close to baseline concentrations within a short period following the race, whilst your excess post-exercise oxygen consumption will continue for a period of hours. Finally, disturbed cardiac autonomic balance are of particular concern, meaning that you essentially bring the stress of the race into the pool with you. Those of you who monitor HRV will be very familiar with a low HRV reading the morning after a late evening high intensity Zwift race!

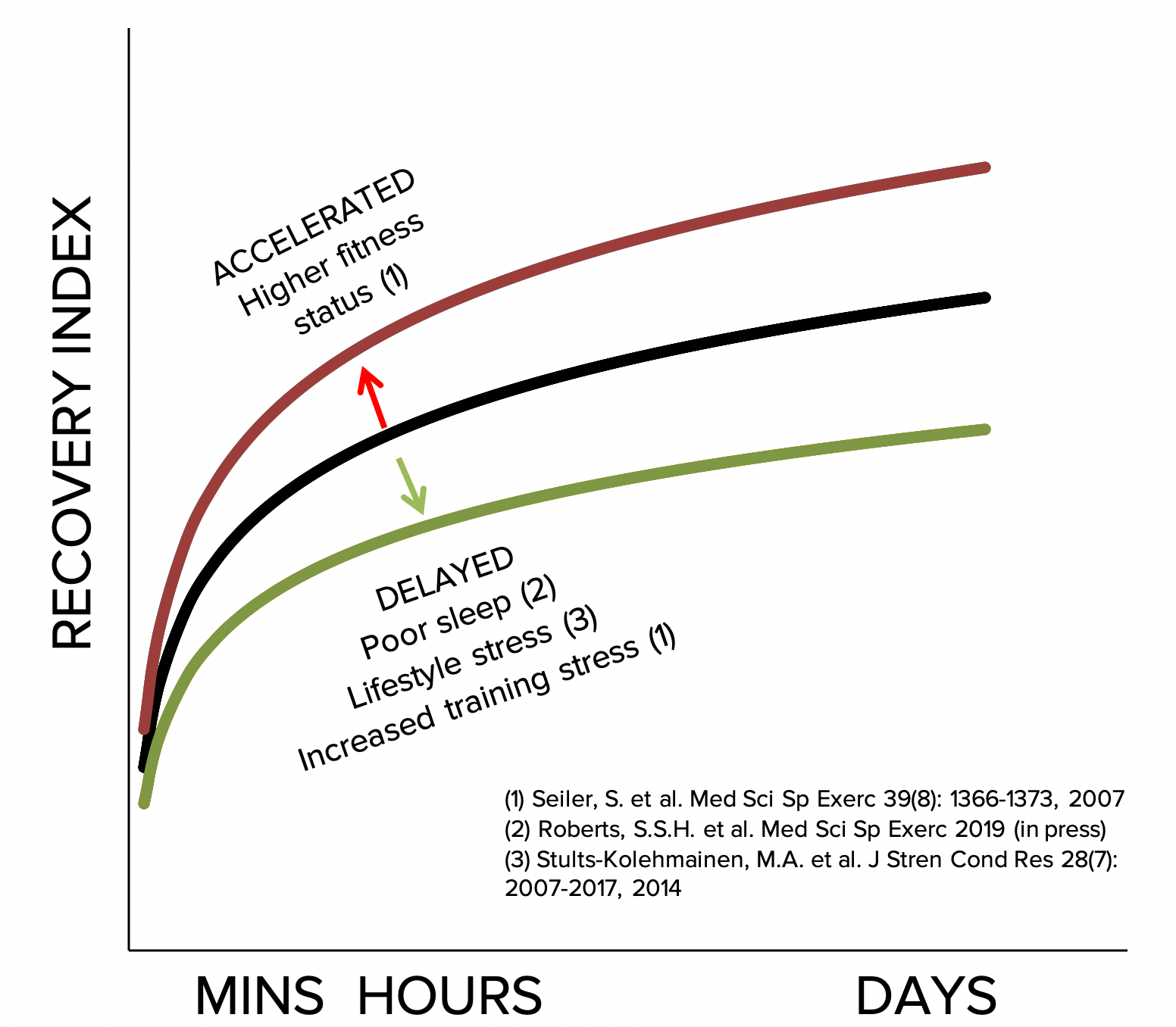
Further complicating the picture are a range of factors that will either accelerate or decelerate the recovery process, and we see these in action with athletes taking HRV measurements all the time (Figure 3). Fitter athletes absorb training easier, and have accelerated recovery from exercise compared to less fit athletes, even if the training is matched for relative intensity (20). Therefore, a 10 x 3-min best effort interval session may take one athlete longer to get over than the same session undertaken by their higher-performing training partner, even though the fitter athlete will have put out more power. Similarly, and as I’m sure you know, poor sleep slows recovery (16), as do other lifestyle stressors (21). Therefore these are factors we should consider not just when deciding whether to do a race or high-intensity training session, but also in our general training process. If we have an acute period of high stress at work we are unlikely to absorb our training as effectively and may need to reduce our overall training load.
Summary: Knowledge is power (and helps with your future power)
Understanding the physiology of recovery, and its time-course in response to different training and racing stimuli, is useful when making day-to-day decisions around training programming. From the above, we have hopefully demonstrated how a spontaneous high-intensity session on a Friday evening will probably leave its mark on an athlete who had planned a fairly hefty subsequent weekend of training, and may impair performance in key interval or long endurance training sessions planned over the subsequent 24-36 hours.
Using this knowledge of the recovery time-course following exercise, as a general rule, we recommend that training performed in the ~48 hours following a demanding session focused above the anaerobic threshold – whether that be a structured interval session or Zwift race – takes place below the aerobic threshold, and is therefore of low-intensity. This strategy is designed such that we nail our key, high-intensity work as best as we can, whilst accumulating low-intensity training volume, and adaptations (9) whilst we are recovering from these efforts.
So, next time you are thinking about jumping into a Zwift race, it might be worth first considering the time-course of physiological recovery from a high-intensity effort like this, and what training you had planned in the subsequent 24-48 hours. In the case of the athlete described here, I’d say that refraining from the Friday night racing in an effort to maximize their outputs in their subsequent planned weekend of training might be the best approach. Thursday may have been a better night for racing, such that enough time was allowed for recovery prior to those planned intervals.
References
- Betts JA, Williams C. Short-term recovery from prolonged exercise. Sport Med 40: 941–959, 2010.
- Bijker JE, de Groot G, Hollander AP. Differences in leg muscle activity during running and cycling in humans. Eur J Appl Physiol 87: 556–561, 2002.
- Billat VL, Demarle A, Slawinski J, Paiva M, Koralsztein JP. Physical and training characteristics of top-class marathon runners. Med Sci Sports Exerc 33: 2089–2097, 2001.
- Bogdanis GC, Nevill ME, Boobis LH, Lakomy HK, Nevill AM. Recovery of power output and muscle metabolites following 30 s of maximal sprint cycling in man. J Physiol 482: 467–480, 1995.
- Cheung K, Hume PA, Maxwell L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sport Med 33: 145–164, 2006.
- Chidnok W, DiMenna FJ, Fulford J, Bailey SJ, Skiba PF, Vanhatalo A, Jones AM. Muscle metabolic responses during high-intensity intermittent exercise measured by 31P-MRS: Relationship to the critical power concept. Am J Physiol – Regul Integr Comp Physiol 305: R1085–R1092, 2013.
- Chidnok W, Fulford J, Bailey SJ, Dimenna FJ, Skiba PF, Vanhatalo A, Jones AM. Muscle metabolic determinants of exercise tolerance following exhaustion: Relationship to the “critical power.” J Appl Physiol 115: 243–250, 2013.
- Desgorces FD, Noirez P. Quantifying continuous exercise using the ratio of work completed to endurance limit associated with exercise-induced delayed-onset muscle soreness. Percept Mot Skills 106: 114–112, 2008.
- Granata C, Jamnick NA, Bishop DJ. Training-induced changes in mitochondrial content and respiratory function in human skeletal muscle. Sport Med 48: 1809–1828, 2018.
- Holt AC, Plews DJ, Oberlin-Brown KT, Merien F, Kilding AE. Cardiac parasympathetic and anaerobic performance recovery after high-intensity exercise in rowers. Int J Sports Physiol Perform 14: 331–338, 2019.
- Laforgia J, Withers RT, Gore CJ. Effects of exercise intensity and duration on the excess post-exercise oxygen consumption. J Sports Sci 24: 1247–1264, 2006.
- Maunder E, Kilding AE, Stevens CJ, Plews DJ. Heat stress training camps for endurance sport: A case study of successful monitoring in two Ironman triathletes. Int. J. Sports Physiol. Perform. .
- McMurray RG, Forsythe WA, Mar MH, Hardy CJ. Exercise intensity-related responses of beta-endorphin and catecholamines. Med Sci Sports Exerc 19: 570–574, 1987.
- Parolin ML, Chesley A, Matsos MP, Spriet LL, Jones NL, Heigenhauser GJF. Regulation of skeletal muscle glycogen phosphorylase and PDH during maximal intermittent exercise. Am J Physiol – Endocrinol Metab 277: E890–E900, 1999.
- Plews DJ, Laursen PB, Kilding AE, Buchheit M. Heart rate variability and training intensity distribution in elite rowers. Int J Sports Physiol Perform 9: 1026–1032, 2014.
- Roberts SSH, Teo WP, Aisbett B, Warmington SA. Extended sleep maintains endurance performance better than normal or restricted sleep. Med Sci Sports Exerc 51: 2516–2523, 2019.
- Ronsen O, Kjeldsen-Kragh J, Haug E, Bahr R, Pedersen BK. Recovery time affects immunoendocrine responses to a second bout of endurance exercise. Am J Physiol – Cell Physiol 283: C1612–C1620, 2002.
- Schumacher YO, Mueller P. The 4000-m team pursuit cycling world record: Theoretical and practical aspects. Med Sci Sports Exerc 34: 1029–1036, 2002.
- Seiler KS, Kjerland GØ. Quantifying training intensity distribution in elite endurance athletes: Is there evidence for an “optimal” distribution? Scand J Med Sci Sport 16: 49–56, 2006.
- Seiler S, Haugen O, Kuffel E. Autonomic recovery after exercise in trained athletes: Intensity and duration effects. Med Sci Sports Exerc 39: 1366–1373, 2007.
- Stults-Kolehmainen MA, Bartholomew JB, Sinha R. Chronic psychological stress impairs recovery of muscular function and somatic sensations over a 96-hour period. J Strength Cond Res 28: 2007–2017, 2014.
- Sylta Ø, Tonnessen E, Seiler S. From heart-rate data to training quantification: A comparison of 3 methods of training-intensity analysis. Int J Sports Physiol Perform 9: 100–107, 2014.








